
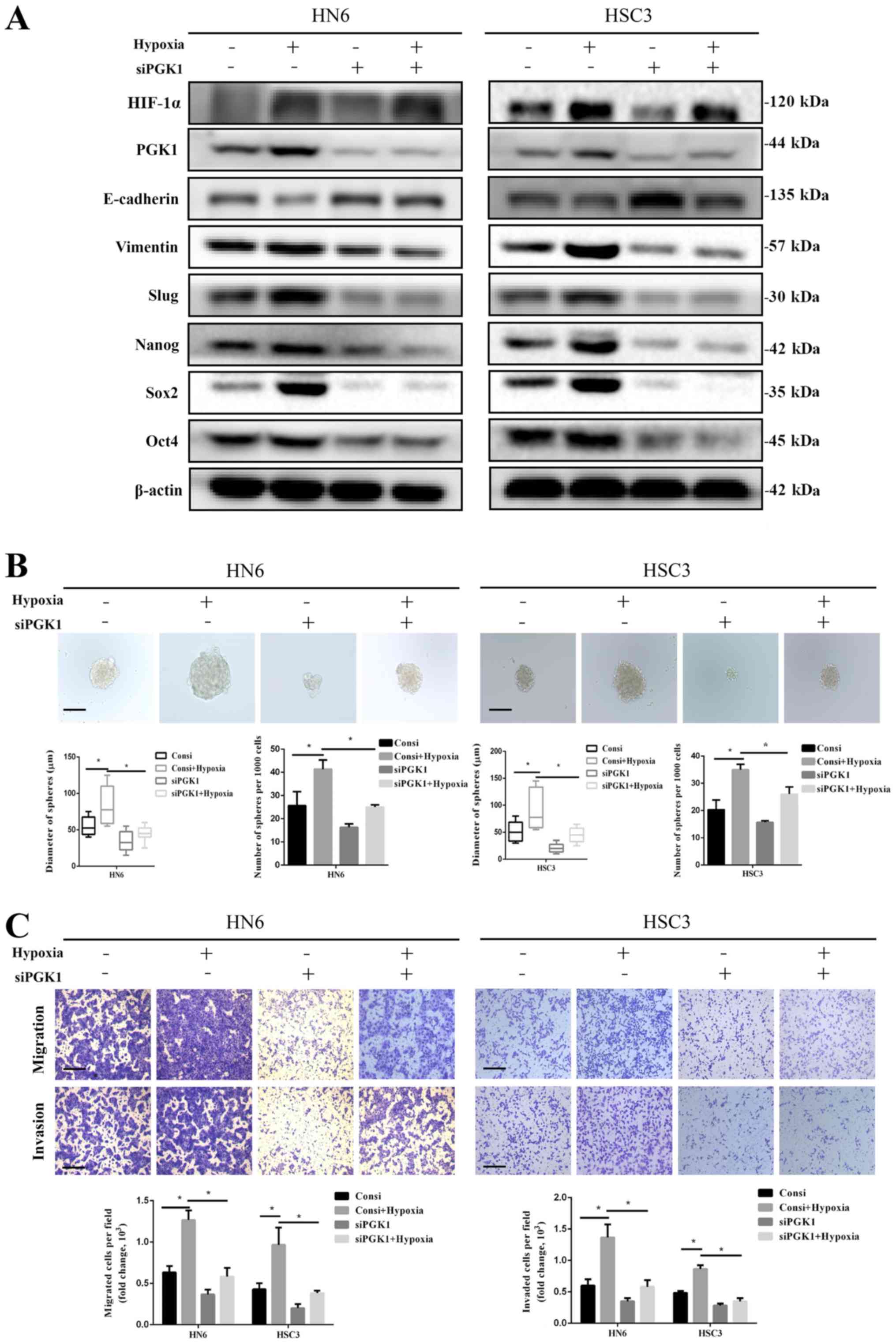
These results suggest a novel model of tumorigenesis, in which PGK1 switches between repressing autophagy-mediated cell death via PRAS40 and inducing autophagy through Beclin1 according to the environmental oxygen level. The binding of PGK1 to PRAS40 was transferred to Beclin1 under hypoxia, resulting in the increase of Beclin1 phosphorylation. Thus PGK1 phosphorylates PRAS40 to repress autophagy-mediated cell death under normoxia, promoting cellular proliferation. By tracing the mechanism, we found that PGK1 suppressed autophagy-mediated cell death, in which PRAS40 was crucial. Both in vitro and in vivo results revealed that PRAS40 mediated PGK1-induced cell growth. PGK1 phosphorylated PRAS40 at Threonine 246, which could be inhibited by blocking the interaction. Herein we screened the binding partners of PRAS40, and found that PRAS40 interacted with Phosphoglycerate kinase 1 (PGK1). However, the detailed role of PRAS40 in autophagy and the relationship to tumorigenesis remain largely unknown. PRAS40 was found to be crucial in various cancers, and phosphorylation was reported to be involved in autophagy inhibition in monocytes. Cell death related to autophagy plays important roles in multiple physiological and pathological situations including tumorigenesis, and the mechanism needs to be defined further. Autophagy predominantly promotes cell survival by recycling cell components, while it kills cells in specific contexts.


 0 kommentar(er)
0 kommentar(er)
